Erythrosin
Name: Erythrosin
CAS Registry Number: 16423-68-0
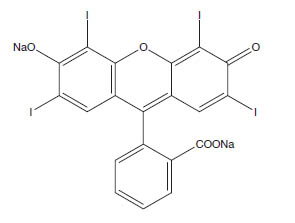
CA Index Name: Spiro[isobenzofuran-1(3H),9'-[9H] xanthen]-3-one, 3',6'-dihydroxy-2',4',5',7'-tetraiodo-, sodium salt (1:2)
Other Names: Erythrosine B; Fluorescein, 2',4',5',7'-tetraiodo-, disodium salt; Spiro[isobenzofuran-1(3H),9'-[9H]xanthen]-3-one, 3',6'-dihydroxy-2',4',5',7'-tetraiodo-, disodium salt; 1427 Red; 1671 Red; 2',4',5',7'-Tetraiodo-fluorescein disodium salt; 2,4,5,7-Tetraiodofluorescein disodium salt; Acid Red 51; Aizen Erythrosine; Aizen Food Red 3; Basovit Red 425E; C.I. 45430; C.I. Acid Red 51; C.I. Food Red 14; Calcocid Erythrosine N; Canacert Erythrosine BS; Ceplac; Cilefa Pink B; Cogilor Red 312.10; D and C Red No. 3; D&C Red No. 3; Dolkwal Erythrosine; E 127; Edicol Supra Erythrosin AS; Edicol Supra Erythrosine A; Erythrosin; Erythrosin B; Erythrosin B sodium salt; Erythrosin BS; Erythrosine; Erythrosine 307046; Erythrosine 36003; Erythrosine 37003; Erythrosine 3B; Erythrosine B-FO; Erythrosine BS; Erythrosine Bluish; Erythrosine Extra; Erythrosine Extra Conc. A Export; Erythrosine Extra Pure A; Erythrosine I; Erythro-sine K-FO; Erythrosine TB; Erythrosine TB Extra; Erythrosine Extra Bluish; Eurocert Erythrosine 311807; FD & C Red No. 3-307020; FD and C Red 3; FD and C Red No. 3; FD&C Red No. 3; FD&C Red No. 3-37003; FDC Red 3; FDC Red 3 dye; Food Color Red 3; Food Dye Red 3; Food Red 14; Food Red 3; Food Red No. 3; Hexacert Red No. 3; Hexacol Erythrosine BS; Japan Food Red No. 3; Japan Red 3; Japan Red No. 3; Maple Erythrosine; Necol Erythrosine; Neelicol Erythrosine; New Ink Bluish Geigy; Red 1799; Red 3; Red No. 3; S 887; Simacid Pink 24107; Spiro[isobenzofuran-1(3H),90-[9H]xanthen]-3-one, 3',6'-dihydroxy-20,40,50,70-tetraiodo-, disodium salt; Synerid; Tetraiodofluorescein sodium salt; Usacert FD & C Red No. 3-310116; Usacert Red No. 3; Water Pink 176575
Merck Index Number: 3693
Chemical / Dye Class: Xanthene
Molecular Formula: C20H6I4Na2O5
Molecular Weight: 879.86
Physical Form: Red to brown powder
Solubility: Soluble in water, ethanol
Melting Point: > 250 °C
pH Range: 2.5-4.0
Color Change at pH: Non-fluorescence (2.5) to light green or reddish fluorescence (4.0)
pKa: 4.1
Absorption (λmax): 525 nm
Emission (λmax): 555 nm
Synthesis: Synthetic methods
Staining Applications: Blood; bone marrow; bacterial plaque; cancer cells; dental plaque; human serum albumin (HSA); lymph node; microorganisms; neurons; nucleic acids; prions; spores; animal feeds; alcohol; baked food; beverages; candies; caramels; confectionery; cotton candy; drinks; fish; olives; orange juices; papaya fruit; soft drinks; sport drink; sweetener; capsules; tablets; sunscreen; eyelids; lips; skin; tattoos; teeth; hairs; keratin fibers
Biological Applications: Detecting gene expression, phosphoproteins, protease, stress biomarkers; treating age-related macular degeneration, arteriosclerosis, bone metabolic diseases, burns, cancer, diabetes, human immunodeficiency virus infection, obesity, viral diseases; medical devices; photodynamic therapy
Industrial Applications: Solar cells; photoelectric device; light emitting diodes; color filters; liquid-crystal displays; thin films; inks; lithographic plate; photographic materials; recording materials; sol-gel materials; photonics; adhesive; paints; thermoplastics; colored bubbles; textiles, entertainment products; toys
Safety / Toxicity: Acute toxicity; carcinogenicity; cytotoxicity; developmental toxicity; DNA-damage; embryotoxicity; genotoxicity; lifetime toxicity; mutagenicity; neurotoxicity; phototoxicity; psychotoxicity; reproductive toxicity
Certification / Approval: Certified by Biological Stain Commission (BSC); Approved by Food & Drugs Administration (FDA)
 Органическая химия. Углублённый курс. Том 2
Органическая химия. Углублённый курс. Том 2 Второй том систематизированного учебного и справочного руководства по ...
 Препаративная органическая химия. Реакции и синтезы в практикуме органической химии и научно-исследовательской лаборатории
Препаративная органическая химия. Реакции и синтезы в практикуме органической химии и научно-исследовательской лаборатории Практическое руководство по лабораторному органическому синтезу, написанное ...
 Методы органической химии. Том 3 выпуск 2
Методы органической химии. Том 3 выпуск 2 Воспроизведено в оригинальной авторской орфографии издания 1935 года ...
 Механизмы реакций в органической химии
Механизмы реакций в органической химии Издание 3-е.По 3-му английскому изданию (1971-г.) Перевод с английского под редакцией ...